Simplifying
The Testing
Supply Chain
For Government, Private Sector
Labs And Testing Service Providers
PCR Testing Supplies
Offering market leading RT-PCR reagents for a wide variety of cyclers, viral lysis buffers for extraction-free processing, specimen collection kits and pipette tips.
Rapid Testing Supplies
Offering a variety of Antigen, Antibody and Titer test kits for field testing with rapid results. We offer test systems either with or without the need for instrumentation.
Welcome To The Future of COVID-19 Testing
Industry Leading RT-PCR Testing, With or Without The Swab.
Market-leading RT-PCR accuracy no matter how you collect the sample.
-
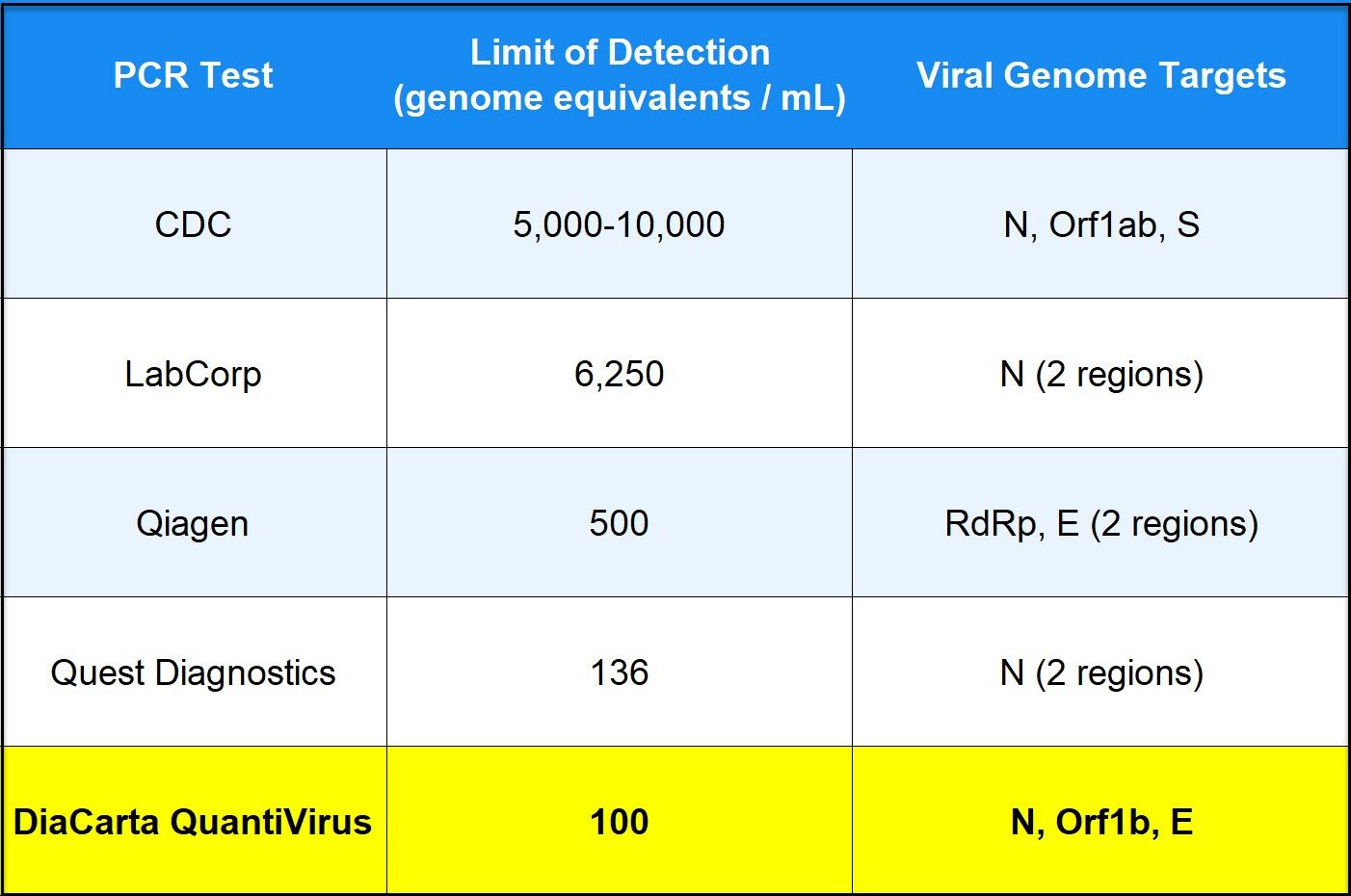
Two different FDA EUA approved reagents with an LOD of 50 or 100 respectively.
-
Two different means of sample preparation: either with or without extraction.
Market Leading Accuracy
In a recent study by the CDC, our PCR reagents were rated among the top 3 assays in the industry. Our greater accuracy means a substantially reduced risk of either false positives or false negatives.
Easy, Safe Sample Collection
While there are many collection kits you can use with our kits, we offer saliva collection kits, oropharyngeal swab kits and nasopharyngeal swab kits. If using our viral lysis buffer for extraction-free processing, you won't even need VTM.
Rapid, Reliable Test Results
Whether performing your tests with or without extraction, the RT-PCR results will complete a plate of up to 384 in just 70 minutes.
The 4-Plex Respiratory Panel
Now offering the DiaCarta 4-Plex Panel, which enables PCR testing for COVID-19, Flu A&B, and RSV. This is a Lab Developed Test (LDT) that employs qRT-PCR technology.
ACCURATE
CONVENIENT
AFFORDABLE
SECURE
A Supplier You Can Count On
Through direct manufacturer partnerships, substantial inventory allocations, and unparalleled client service, BioLux has become a supplier that public and private sector as well as labs, retailers and testing providers have come to trust. We can help with product selection and deliver wholesale testing supplies to clients large and small. Give us a call today.
FAQs
-
WHERE ARE YOUR KITS WAREHOUSED?
Each product varies with regard to warehousing. While we're a stocking distributor for some of our products lines, others are drop shipped factory direct. Please inquire for the particular products(s) you are interested in.
-
WHERE'S MORE INFO ON PCR REAGENTS?
For additional information on the Dia Carta reagents, please click HERE.
-
DO THE COVID REAGENTS PRODUCTS HAVE EUA?
Yes the COVID assays we offer do have Emergency Use Authorization (EUA) from the FDA.
-
HOW LONG DOES DELIVERY TAKE?
Delivery times will vary by product and order volume. PCR reagents take 1 day to leave our California warehouse and are shipped overnight.
-
HOW ARE PCR SAMPLES COLLECTED?
Collection may be completed with oral, nasal or nasopharyngeal swabs as well as via saliva collection. For saliva collection, the test subject holds a plastic vial in their hand, spits into the vial and closes the vial cap. Click HERE to view the instructions and click HERE to view video instructions.
-
WHAT'S THE MINIMUM ORDER QUANTITY?
MOQ varies by product, from as little as a single OTC test kit to multiple pallets. Please contact us directly to inquire.
-
HOW DO WE PAY FOR ORDERS?
Most orders are paid for via eCheck, ACH or Wire Transfer.
-
WHAT OTHER PRODUCTS DO YOU OFFER?
In addition to reagents and test kits for COVID, we offer all other products by our same suppliers, including products such as respiratory panels, UTI panels, cancer panels, tox screening products, gloves, pipette tips and more.
Still Have Questions?
Ready To Get Started?
Let us get you the information you need.
Legal Disclaimer: The following applies to ALL COVID-19 testing products on the market which have been authorized by FDA under an EUA, including those offered through BioLux:
This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA for use by authorized laboratories; This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
© 2020 BioLux
